
How generative AI is reducing drug development timelines
Did you know that the journey for a groundbreaking drug from concept to market spans a staggering 12 to 18 years, with a jaw-dropping price tag of $2.6 billion? What’s more, only a tiny 10% of candidates even make it to clinical trials. But here's the good news–Generative AI is stepping in to rescue the day for pharma execs.
These real-life examples drive the point home:
- Absci has harnessed its power to design antibodies that target various disease-associated molecules including (HER2 receptor, VEGF growth factor, and spike protein of SARS-CoV-2). Hold onto your lab coats because this groundbreaking technology could potentially slash drug discovery timeframes by up to a whopping 50%.
- Insilico Medicine has birthed a game-changing generative adversarial network-based AI platform. They engineered the world's first AI-forged anti-fibrotic small molecule inhibitor and propelled it into the realms of Phase II clinical trials.
- Adaptyv Bio, the Swiss biotech leader, pioneered a generative AI platform that's redefining protein engineering, leveraging generative algorithms, robotics, microfluidics, and synthetic biology as their weapons of choice.
- Iktos and Curreio are on a mission to revamp drug discovery by unleashing generative AI and cryo-electron microscopy (EM) technology to increase the accuracy of predicting molecules that meet specific product profiles.
Soon, we’ll be living in a world where groundbreaking medicines hit your local pharmacy faster than ever, all thanks to the potential of Generative AI. It's not science fiction—it's the future of healthcare.
Generative AI applications in drug R&D and discovery
An in-depth look at how Generative AI is revolutionizing the entire drug R&D and discovery lifecycle with many powerful applications will help you take that crucial first step toward integrating this technology into your research lab. In this blog, we'll uncover how generative AI can optimize:
- Drug discovery, formulation, and repurposing;
- Experiment tracking and record-keeping;
- R&D trial tracking & smart materials/chemical search; and
- Clinical trial feedback summarization.
Drug discovery, formulation, and repurposing
While strides have been made to streamline drug discovery, formulation, and repurposing, these steps continue to present intricate challenges in pharmaceutical research. Despite notable reductions in timescales, complexity, and costs, as well as improved precision, there is still ample room for advancement. Enter Generative AI, a transformative force that holds the potential to reshape these pivotal stages. Through intricate analysis of biological data, AI algorithms skillfully identify disease targets, predict interactions with drug contenders, and optimize developmental paths. The synergy of machine learning elevates experimental design, predicts drug behaviors, and ushers in the era of personalized medicine.
Compound generation and virtual screening
AI generates novel chemical compounds for disease targets, exploring vast chemical spaces to propose unique molecular structures for further investigation that bind to proteins or other molecules indicative of viruses and bacteria. Generative AI-based virtual screening predicts the binding affinity of generated compounds to target proteins using molecular docking simulation, prioritizing compounds with desired biological activity. This tandem approach accelerates drug discovery, especially when targeting complex diseases with tailored molecules.
Generative AI constraints for compound generation
Constraints of generative AI in compound generation include lead optimization for enhancing potency and predicting ADME properties to improve clinical trial success rates.
- Lead optimization: Generative AI can suggest modifications to existing compounds to improve their potency, selectivity, and pharmacokinetic properties, guiding the lead optimization process.
- Prediction of ADME properties: Generative AI models can predict the absorption, distribution, metabolism, and excretion (ADME) properties of compounds, aiding in selecting candidates with better chances of success in clinical trials.
Compound generation approaches
Diverse approaches of generative AI in compound generation include De Novo Design for novel compounds and predicting protein structures to unravel their functions and interactions.
- De Novo Design: In drug discovery, De Novo Design involves using computational methods and algorithms to generate novel chemical compounds that are optimized for desired properties or functions such as binding to a particular target protein or exhibiting specific biological effects. This approach is valuable for exploring chemical space beyond what is available in existing databases and can lead to the discovery of new drug candidates with unique properties.
- Protein structure generation: By predicting the physical structures of proteins through the spatial positions of amino acids that make the protein, pharma companies can understand its function and interaction with other molecules. For example, the physical structure of a protein can determine its properties, including whether it's soluble (dissolvable in water) or non-soluble, its stability, and how it interacts with other molecules.
Formulation optimization
Generative AI's potential in formulation optimization includes opportunities for solubility prediction, excipient selection, dosage form design, process enhancement, polymer design for controlled-release formulations, and stability prediction.
- Solubility prediction: Generative AI can predict the solubility of drug candidates in various solvents, enabling formulation scientists to choose the best solvent for drug delivery.
- Excipient selection: By selecting appropriate excipients, generative AI models can enhance drug stability, bioavailability, and patient acceptability.
- Dosage form design: Generative AI models can suggest optimal dosage forms such as tablets, capsules, or injections based on the physicochemical properties of the drug and the desired release profile.
- Process design: Generative AI can be used to improve compound generation processes by analyzing current and historical drug discovery processes to suggest optimized steps.
- Polymer design: For controlled-release formulations, generative AI analyzes historical data of the previous compounds, and factors such as solubility and coating are scrutinized for improvement. For instance, on average, a 1mm coating led to a 4-minute system response time. Generative AI leverages this data to formulate polymer matrices that precisely regulate drug release kinetics, optimizing efficacy and patient experience.
- Stability prediction: Generative AI can predict the stability of drug formulations under different conditions, guiding researchers to design formulations that remain effective over time.
Example:
Researcher: "I need a novel compound targeting protein XYZ for cancer treatment."
Generative AI: "Based on your input, I have generated a set of 100 novel compounds with predicted binding affinities to protein XYZ. Here are the top 10 candidates, along with their predicted ADME properties. Would you like to prioritize any of these compounds for further testing?"
Drug repurposing
Generative AI's potential in drug repurposing ranges from database mining for efficient candidate identification to network analysis of molecular interactions, revealing novel connections between drugs and diseases. Additionally, generative AI's exploration of off-target effects opens doors to repurposing existing drugs for treating diverse medical conditions, expanding therapeutic possibilities
- Database mining: By analyzing extensive drug compound databases, generative AI can identify potential candidates for repurposing, speeding up the search process. For example, generative AI models might discover that a drug intended initially for hypertension could also effectively treat a neurodegenerative disease.
- Network analysis: Generative AI conducts network analysis of molecular interactions, unveiling connections between drugs and diseases that might not be apparent through traditional methods. For instance, Generative AI might reveal that a drug used for a specific cancer could also target a different pathway in a rare genetic disorder.
- Off-target effects: By examining drug side effects, generative AI uncovers off-target effects that could be repurposed for treating different medical conditions, enhancing therapeutic possibilities. For example, generative AI could identify that a drug's known side effect of suppressing inflammation might be beneficial for an autoimmune disorder.
Efficient experiment tracking and record-keeping
At any given time, a pharmaceutical company could be dealing with tonnes of information related to 100-150 ongoing drug discovery experiments. Using text and image-based models, generative AI helps automate experiment tracking by generating summaries or reports of these experiments. This can help medical researchers keep better records and track progress more efficiently.
Automated summaries
Through text and image-based models, generative AI crafts concise summaries covering experiment objectives, methodologies, results, and observations. It significantly reduces manual documentation efforts, ensuring meticulous record-keeping.
Example:
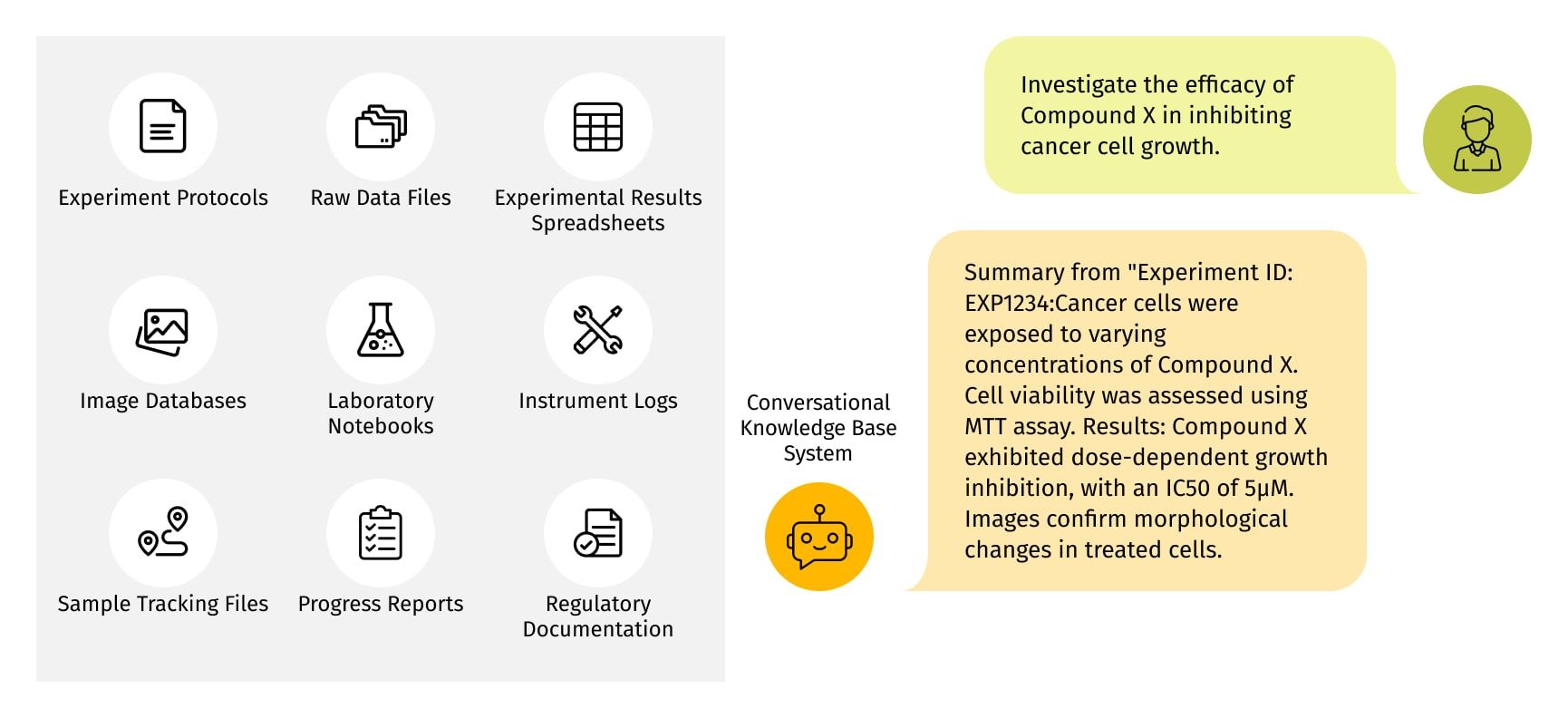
Extracting key highlights
Generative AI, with its analytical prowess, identifies pivotal findings and emerging trends within experiment data. These highlighted insights empower researchers to swiftly grasp and compare outcomes across experiments, facilitating informed decision-making.
Example:
Generated Highlights: "Emerging Trend: Compounds containing halogen substituents consistently showed higher anticancer activity across experiments. This pattern suggests a potential avenue for enhanced drug design."
Complex query handling
Researchers can seamlessly interact with a generative AI interface, posing intricate queries about experiment progress. Generative AI's capacity to interpret complex questions empowers researchers to inquire about experiment status, pending tests, overall conclusions, and even specific aspects like toxicity and solubility of drugs.
Example:
Reference Data: Toxicity and solubility data from ongoing experiments.
Generated Response: "Toxicity: Experiments revealed low toxicity levels, with no significant adverse effects observed at tested concentrations. Solubility: Most formulations displayed improved solubility, with Compound Y's solubility increasing by 40% in pH-neutral media."
Efficacy insights
Beyond generating summaries, generative AI offers invaluable insights by identifying potential avenues for cost reduction and efficacy enhancement. This capability proves especially impactful in the pre-clinical trial phase, aiding researchers in optimizing resources.
Example:
Generated Insights: "Resource Optimization: Generative AI identified Experiment X's cost inefficiencies due to excessive reagent consumption. Suggests exploring alternative protocols to minimize expenses.
Efficacy Enhancement: Compound Z demonstrated 20% higher efficacy in 48-hour exposure compared to 24-hour exposure. Recommends focusing on longer exposure times for improved results."
Virtual assistant for R&D: Trial tracking & smart materials/Chemical search
A virtual assistant fueled by Generative AI for pharmaceutical drug R&D promises a wealth of transformative benefits for R&D teams. This R&D assistant serves a dual role with remarkable proficiency. Firstly, it empowers researchers to maintain real-time access to the latest clinical trial data, facilitating swift and informed actions that ensure the safety and efficacy of ongoing trials while also providing essential insights for future drug discovery initiatives. Simultaneously, it acts as a powerful tool for conducting intelligent and highly efficient searches for materials and chemicals, driven by specific desired properties and criteria.
Access to real-time clinical trial data
Generative AI's impact on real-time clinical trial data offers opportunities ranging from real-time status updates and data summarization to providing recommendations for the next best actions based on historical trial data. Additionally, the assistant ensures timely alerts and notifications to researchers, facilitating prompt actions in response to specific conditions or events during the trial.
- Status updates: Researchers can query the assistant to get real-time updates on the status of ongoing trials, including enrollment numbers, key milestones, and adverse event reports.
- Data summarization: The assistant can generate concise summaries of trial progress, highlighting significant findings, patient responses, and potential areas for further investigation.
- Next best action recommendation: Based on historical trial data, the assistant can suggest the next best steps for trials, identifying potential adjustments in dosage, patient demographics, or monitoring strategies.
- Alerts and notifications: The assistant can send automated alerts or notifications to researchers when specific conditions or events are met during the trial, ensuring prompt action.
Smart materials/chemical search
Generative AI's impact on smart materials and chemical search offers opportunities such as compound recommendation, structural similarity search, property prediction, property assessment, literature review, and semantic vector search. This section explores how AI aids researchers in efficient material and chemical exploration, prediction, and literature analysis.
- Compound recommendation: Researchers can describe the desired properties of a compound, and the assistant can suggest a list of potential materials or chemicals that match the criteria.
- Structural similarity search: The assistant can perform searches based on structural similarity to known compounds, helping researchers identify potential analogs with similar properties.
- Property prediction: The assistant can predict the properties of new materials or chemicals using machine learning models, aiding in early-stage screening.
- Property assessment: Generative AI aids in identifying or retrieving chemicals with desired properties, including toxicity and solubility characteristics, by analyzing data and producing suggestions, streamlining focused chemical selection.
- Literature review: The assistant analyzes scientific literature to gather relevant information about the materials or chemicals being considered, saving researchers time on manual literature searches.
- Semantic vector search: Utilizing semantic vector search technology to understand the contextual meaning of queries, the assistant provides relevant search results going beyond keyword matching, accounting for the semantic relationships between words.
The new semantic search delivered by Grid Dynamics includes several essential capabilities:
- Multi-stage concept-oriented search for automated balancing of precision and recall;
- Query expansion with domain-specific knowledge graphs;
- Intent classification;
- A query understanding system based on the graph representation of a query.
Text-based clinical trial feedback summarization
Did you know that clinical trial researchers spend a lot of time post-trail finding meaningful insights from extensive quantitative and qualitative data from participants, investigators, and stakeholders? Generative AI-powered text-based feedback summarization is the game-changer. It efficiently processes vast datasets, extracting key insights, trends, and sentiments. These are then condensed into concise summaries, providing researchers with a quick overview of overall sentiment, common themes, and critical measurements. This streamlines data analysis and enhances decision-making in clinical trials. Below are some patient experience summarization examples.
Example:
Text feedback analysis
Consider patient text feedback regarding the trial's effects. Generative AI identifies recurring keywords and sentiments, highlighting positive or negative experiences.
Example:
AI Summary: "Positive impact observed on pain reduction and sleep quality improvement."
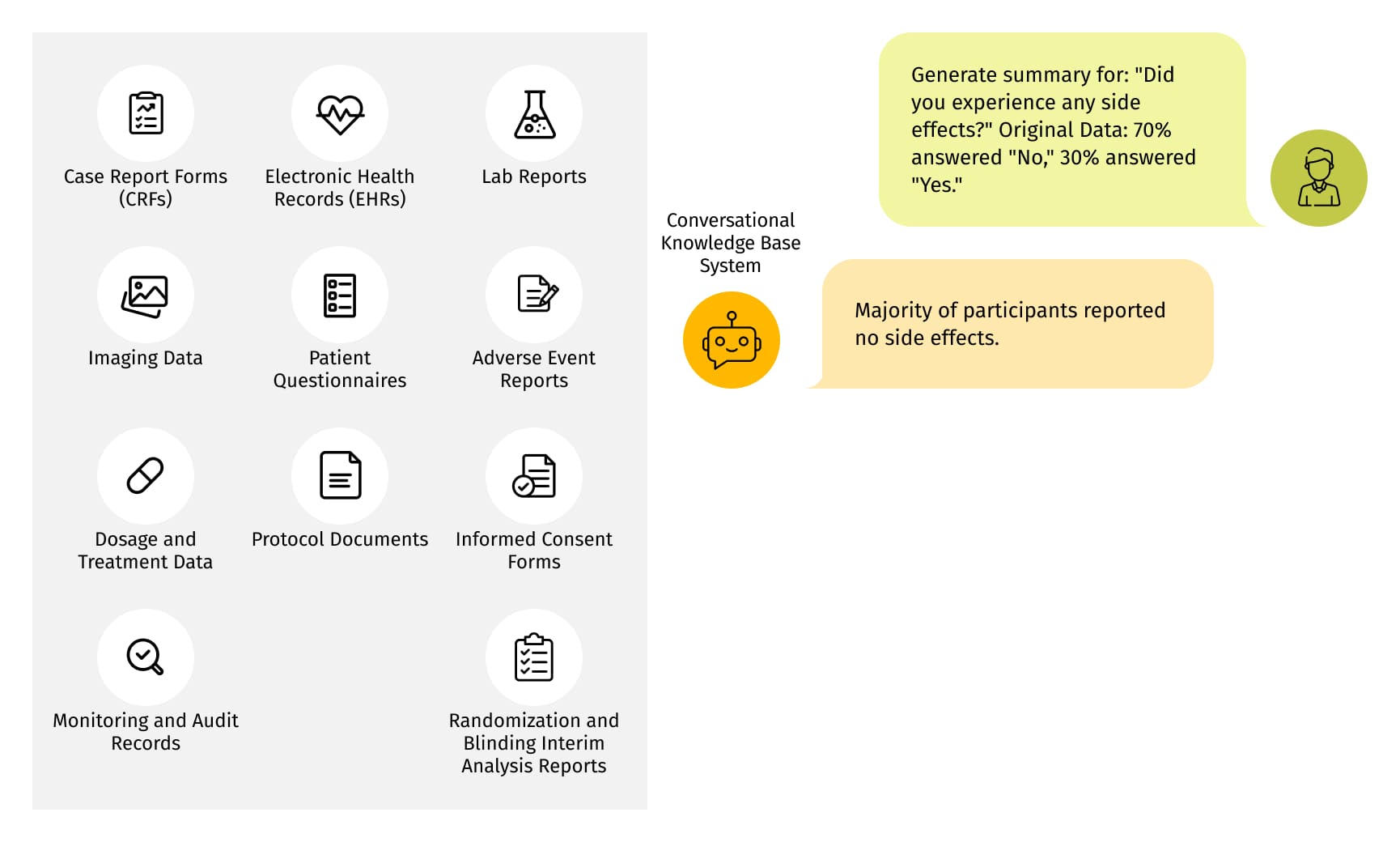
Yes/No questions
AI processes binary responses to questions such as "Did you experience any side effects?" and generates summarized statistics.
Example:
AI Summary: "Majority of participants reported no side effects."
Blood test and clinical measurements
The AI can analyze various clinical measurements, identifying trends and anomalies.
Example:
AI summary: "Group A exhibited a significant average blood pressure reduction of 10 mmHg."
Temporal analysis
Generative AI can track changes over time.
Example:
AI summary: "Pain score decreased by 20% from baseline after Week 4."
Adverse events
Generative AI can summarize clinical trial adverse events.
Example:
AI summary: "Adverse events included skin rash in Patient X and dizziness with blurred vision in Patient Y."
Investigator feedback and next best experiments (NBEs)
Generative AI can provide investigator feedback summaries and recommend next best experiments.
Example:
AI summary: "Investigators suggested protocol adjustments to accommodate patient demographics and noted issues with unreliable monitoring equipment."
AI-Generated NBE: "To further compare the new drug's effectiveness against the standard treatment, the Next Best Experiment could involve a longer-term follow-up to track sustained outcomes over an extended period."
Challenges in leveraging generative AI for drug discovery
Undoubtedly, the integration of generative AI in drug discovery holds immense promise, but it also presents a complex web of challenges. To fully unlock generative AI's potential in this domain, we must proactively tackle these obstacles. This necessitates efficient collaboration among a multitude of stakeholders, including scientists, regulatory bodies, and the pharmaceutical industry. The objective is to establish robust best practices and standards while effectively navigating the legal, ethical, and scientific complexities inherent in drug discovery.
High-quality data requirements
Generative AI models demand extensive and precise datasets to make informed predictions. In drug discovery, this translates into a need for comprehensive molecular data, patient profiles, and clinical trial results. For example, to develop a new cancer drug, pharmaceutical companies require vast and accurate genomic data from patients. Incomplete or erroneous data could lead to inaccurate predictions and failed experiments.
Ensuring AI interpretability
AI models often operate as "black boxes," making it challenging for scientists to comprehend how they arrive at specific conclusions. If a generative AI model suggests a particular molecule as a potential drug candidate, scientists need to understand the molecular interactions or biological pathways that led to this recommendation. Generative AI might identify a compound as a potential drug candidate due to its molecular structure and binding affinity to a specific protein target. Understanding these underlying mechanisms is crucial for validating the AI's predictions.
Regulatory compliance
The pharmaceutical industry is heavily regulated to ensure patient safety. Adhering to regulatory requirements when implementing generative AI in drug discovery is a multifaceted challenge. The FDA has stringent criteria for approving new drugs, including thorough testing and documentation. Demonstrating that generative AI-driven research complies with these regulations is a significant hurdle. Pharmaceutical companies must ensure that their generative AI models meet regulatory standards and can provide the necessary documentation for the approval of new drugs. This often involves extensive testing and validation to demonstrate safety and efficacy.
Ethical considerations
Ethical issues arise concerning data usage, informed consent, and the potential consequences of generative AI-driven decisions on patients' well-being. Using patient data for drug discovery must adhere to strict ethical guidelines, ensuring data privacy and patient consent. Pharmaceutical companies should establish robust data governance policies that include anonymizing patient data, obtaining informed consent, and ensuring that generative AI-driven research does not harm patients' interests.
Resource allocation
Implementing generative AI in drug discovery demands substantial investments in computing infrastructure, skilled personnel, and data acquisition. Setting up a high-performance computing cluster for generative AI-driven molecular simulations requires a significant financial commitment. Pharmaceutical companies must allocate resources effectively to build the necessary infrastructure and hire generative AI experts while ensuring that they have access to high-quality data for training their generative AI models.
Integration with traditional methods
Generative AI should complement, not replace, existing drug discovery methods. Ensuring a smooth integration can be a complex task. Traditional wet lab experiments must align with generative AI predictions to validate the efficacy of potential drug candidates. Pharmaceutical companies need to create workflows that seamlessly integrate generative AI predictions with laboratory experiments. For example, if a generative AI model identifies a promising drug candidate, it must be tested in the laboratory to confirm its effectiveness.
Data security
Handling sensitive patient data and proprietary research information raises concerns about data security and privacy. Protecting patient data during generative AI-driven clinical trials is paramount to prevent data breaches and privacy violations. Robust cybersecurity measures are essential to safeguard patient data and proprietary research findings. This includes encryption, access controls, and regular security audits to identify and mitigate vulnerabilities.
Expertise bridging generative AI and life sciences
Bridging the gap between generative AI experts and life scientists is essential for effective collaboration. A generative AI researcher might recommend a specific molecule for further study, but life scientists need to understand its biological relevance and potential side effects. Cross-disciplinary training programs can help researchers in both domains understand each other's language and work collaboratively. Life scientists can benefit from courses that teach the basics of generative AI and data science, while generative AI researchers can gain insights into the nuances of biology and chemistry.
Addressing these challenges requires synergistic efforts:
- Collaborative research: Encourage collaboration between pharmaceutical companies, generative AI researchers, and regulatory bodies to establish data-sharing standards and best practices.
- Ethical frameworks: Develop ethical guidelines for using patient data in drug discovery and ensure that generative AI-driven decisions prioritize patient safety and informed consent.
- Resource planning: Allocate resources judiciously by identifying areas where generative AI can have the most significant impact and focusing investments there.
- Interdisciplinary training: Promote educational programs that facilitate collaboration between generative AI experts and life scientists, ensuring that both sides understand each other's needs and constraints.
- Transparency and interpretability: Invest in research to make generative AI models more interpretable, ensuring that scientists can trust and validate generative AI-driven recommendations effectively.
These efforts can come to fruition when generative AI projects in pharma transition from experimental silos to strategic and holistic components of the organizational roadmap. This means fully integrating into the pharma ecosystem, adopting AI tools, nurturing talent, staying compliant, mastering data management, and measuring the return on investment (ROI).
Conclusion
Pharma executives, the future of drug development is at your doorstep. Say goodbye to those never-ending timelines and exorbitant costs.
From automating experiments and streamlining R&D decisions to providing real-time updates and intelligent material searches, generative AI is the key to an era of efficiency. It revolutionizes compound generation and drug repurposing, suggesting the next best experiments, predicting properties, and optimizing formulations. It even ensures the stability of your drug formulations. But its impact goes far beyond—generative AI finds applications in drug manufacturing, regulatory compliance, competitor research, strategic investments, technology modernization, and more.
Unlocking the full potential of generative AI requires collaboration. It's a synergy between healthcare systems, research groups, and tech leaders. Grid Dynamics, with its seasoned data science and AI teams, is your strategic partner. We offer optimized data management, AI-driven anomaly detection, agile analytics, personalized medicine, and next-best actions to streamline pharma operations and elevate patient care.
The revolution of generative AI in pharma and life sciences has begun, promising innovative discoveries, patient-centric care, and operational efficiency. Choose Grid Dynamics as your agile co-innovation partner, and together, we'll unleash the full potential of generative AI for healthcare.
