
KPIs for pharmaceutical manufacturing operations
Increasing customer expectations, disruption of operations, and intense competition have placed the entire manufacturing industry under pressure. Pharma manufacturing, in particular, faces extremely complex supply chain management processes that require commercial pharma companies to monitor and understand performance indicators that impact the manufacturing operations’ value chain.
This article provides a KPI-centric view of pharmaceutical manufacturing operations, and explores the following topics:
- how to break down the "100% order fulfillment on time" goal into specific manufacturing metrics to monitor;
- which supply chain reliability metrics are crucial to support the above goal;
- how reliability can be mapped into more granular metrics of supply management and deviation minimization;
- why worker safety and compliance are foundational, and what to consider when tracking them; and
- how to use technology to streamline planning and decision-making based on the gathered data.
You control what you measure: pharma manufacturing metrics worth tracking
The worldwide value of the pharmaceutical goods trade has grown from approximately 110 billion dollars in 2000 to roughly 630 billion dollars in 2019. This amazing growth implies that pharmaceutical supply chains have become increasingly global and complex and that the underlying manufacturing processes are under increasing pressure.
Due to demand-supply complexity, challenges with value chain intermediaries, and multiple regulatory requirements, pharmaceutical companies focus on achieving "100% order fulfillment on time"—or Right First Time (RFT)—as one of the most important manufacturing performance metrics. That is why accurate measurement is a crucial component for improving operational performance and, thus, maintaining competitive advantage in a continuously changing environment (Figure 1).
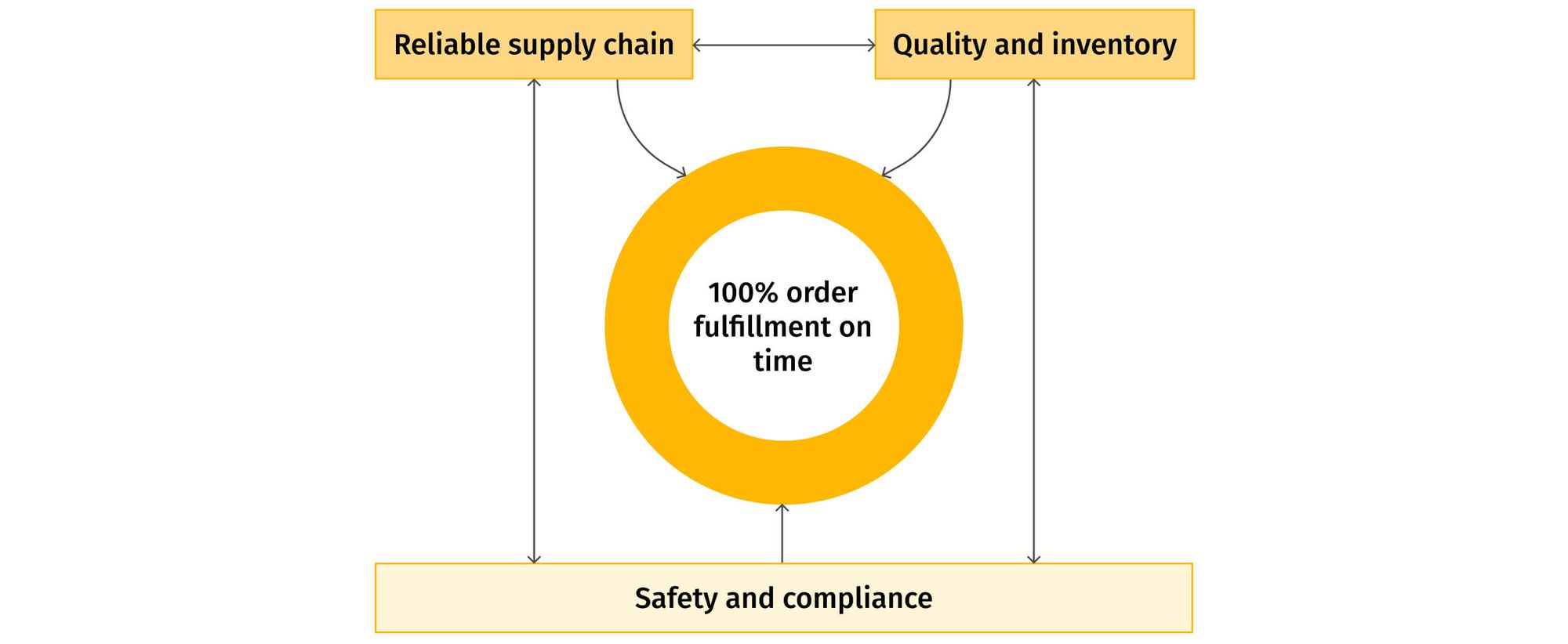
Measure toward the Right First Time delivery goal
Since one of the primary objectives of pharma manufacturing is 100% order fulfillment on time and in the right quantity, Right First Time (RFT) is a crucial goal to keep in mind when measuring the effectiveness of process KPIs. With production costs typically being only a fraction of the total price, it is important to concentrate on the on-time delivery of the full quantity of good quality products as the primary objective function of supply chain activities.
To achieve this ambitious RFT manufacturing goal, the KPIs to measure are:
On Time to Request—the ability to deliver products in full on or before the date requested by the customer.
On Time to First Promise—the ability to meet the shipment date committed by the company to meet the requested quantity in full.
On Time in Full (OTIF)—the order fulfillment accuracy. The OTIF ratio is calculated by comparing the number of orders that were fulfilled accurately (on time and in the right quantity) to the number of all orders.
Months of Supply—on-hand inventory available compared to the service demand for a defined time period.
Tracking these KPIs for manufacturing operations provides the level of transparency needed to have actionable data for:
➔ Product line and warehouse operations optimization
➔ Logistics (inbound and outbound)
➔ Quality and dynamic process control
Assess supply chain reliability
Reliable and stable supply chains power the RFT manufacturing goal. Overall equipment effectiveness (OEE) is an applicable key measure for operationalizing and evaluating supply chain reliability metrics.
For pharmaceutical manufacturing, the reliability of the supply chain means having multiple dependable sources of all the raw materials and manufacturing equipment that are available for production. In this regard, using OEE as a KPI enables production centers to monitor and improve the productivity of a machine or a production line.
OEE (calculated as Availability × Performance × Quality) provides a way to classify and calculate the efficiency vs. losses during drug production.
The key KPIs to be examined are:
DS/DP yield, where
- DS = drug substance (an active pharmaceutical ingredient that contains potent compounds); and
- DP = drug product (a packaged form of the actual medicine for the consumer).
Output/Lot—the mass of drug substance (or vials or pills) per manufacturing run. The auxiliary KPIs to track under this category are yields, lead times, release time, and deviation rates.
Output/Day—the mass of drug substance (or vials or pills) per day.
Auxiliary KPIs to track under this category are cycle times and plant uptime.
Quantify the quality of the process
In addition to the production metrics defined earlier, it is important to measure the verifiable quality of the lot being produced. The main driver is, of course, meeting and exceeding the manufacturing standards of the drugs marketed in a certain region (such as the FDA’s Current Good Manufacturing Practices or EudraLex’s Good Manufacturing Practices guidelines), and reducing shortages and product recalls.
Moreover, minimizing lot failure rates helps free up production capacity and avoids rework and stoppage. The key KPIs to monitor are:
Leading indicator, process capability index—the measure of the process’s ability to produce outputs within the specifications; and
Lagging indicator, process performance index—the measure of the actual outputs within the specifications.
Other metrics to be considered are error percentage in documents across processes caused by manual entries, which lead to quality-related delays.
In addition to thorough drug quality control practices, measuring lot failure rates is key to minimizing deviations and increasing quality control for pharmaceutical manufacturing processes.
Estimate safety and compliance costs
While material costs during the production process might not be that significant, human-related costs are important to consider. They impact utilization rates, which are reflected in scheduling, and might then be impacted due to worker safety and compliance issues.
The related KPIs to monitor and act upon include:
- Injuries
- Lost workdays
- At-risk behaviors
- Violations (for example, OSHA injury and illness recordkeeping and reporting requirements)
Summary: the data goldmine
Digital transformation and advanced technologies, from data science and AI to IoT, are major enablers that help increase transparency and support companies at large with insightful intelligence. On top of that, they streamline the application of the “experience > intelligence > value > innovation” path across drug manufacturing, and minimize supply risk.
Analyzing the data with AI-powered solutions enables a combination of real-time insights with historical, market, financial, and geopolitical data to make evidential decisions for optimizing plant performance. Noteworthy production opportunities include improving line saturation, reducing downtime, preventing equipment failure, improving drug product quality, and optimizing raw material stock.
References
- UN Comtrade Database, Feb 2020 | United Nations
- Current Good Manufacturing Practice (CGMP) Regulations | FDA
- EudraLex, Volume 4, Good Manufacturing Practice (GMP) Guidelines | European Commission
- OSHA Injury and Illness Recordkeeping and Reporting Requirements | Occupational Safety and Health Administration
